Tamarindus Indica as a potential solid polymer electrolyte material for Sodium Batteries; Kaushik P. V. N. M., N. K. Jyothi, M. S. Jogad, Akhilan S. R., R. Menon; Vol. 2, Issue 1, (January) 2025.
Authors: Kaushik P.V.N.M.*, Awareness 2025, 2(1): 30-41
- Jan 04, 2025
- 201 views

Abstract
The role of energy storage devices in the modern-day world is phenomenal. Though lithium-ion batteries have ruled the market for several decades, few constraints have surfaced over the safety offered by them. Thus, an alternative choice of storage devices served the purpose of overcoming the setbacks of lithium batteries. The concept of polymer electrolyte is one of the greatest inventions in the field of energy storage. The invention of solid-state batteries revolutionized the battery technology over the past century. From the choice of using synthetic polymers for the preparation and fabrication of these devices, research studies have evolved to discover another class of novel materials known as ‘biopolymers’, which exhibit similar results as in the case of synthetic polymers, in terms of energy efficiency. Among the several reviews reported on the usage of several biopolymers in the application of energy storage devices, Tamarind Seed Polysaccharide (also known by the name Tamarindus indica) is one such novel biopolymer which is currently being used in the preparation of electrolyte materials for storage devices. This paper seeks to explore the similarities of biopolymers, in particular Tamarind seed polysaccharide (TSP), and discuss how its properties are similar to those exhibited by synthetic polymers, thereby concluding that bio-materials may be preferred over synthetic materials. The preference of novel biopolymers such as TSP for application in sodium batteries is discussed towards the end of this paper. The present research study seeks to establish the feasibility of application of biopolymers such as TSP in sodium-based batteries.
Full Text
1. Introduction
Since the industrial revolution, the use of energy, particularly electrical energy, has become the need of the hour. Over several decades, research studies were performed in order to make energy more accessible to all sectors of humanity
Apart from the need for energy devices with enhanced efficiency, researchers also came up with novel ideas in order to store the produced energy and make it portable [1]. The exhausting resources of fossil fuels was never a constraint for modern man as he found suitable alternatives to replace fossil fuel resources. Among the various storage devices that were discovered and fabricated to suit the needs of modern man, batteries found their place on the top [2]. The thought that “modern day battery is a miracle” is never exaggerating, as the wide usage of batteries is seen across the spectrum, be it industry, academics, pharmacy, transport sector, etc. [3].
The evolution of batteries in the 19th century crossed various stages such as nickel cadmium battery, lead acid battery, nickel metal hydride battery etc. and finally landed at the well-known “Lithium-ion” batteries.
2. Genesis and developments in Lithium – ion batteries
Scientific studies over the past few decades lead to the conclusion that the element Lithium or Li (period 2, group 1 of the modern periodic table) exhibits several essential characteristics befitting a battery element [4]. Being a light metal with low density and lower value of standard reduction potential makes lithium a suitable material to prepare high-voltage batteries with higher densities. In the book “The Nobel Prizes 2019”, Grandin [5] gives a detailed description of the latest stages in the evolution of the lithium-ion batteries (LIBs). He states that “the discoveries of John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino have arguably had a tremendous impact on our world” (these 3 people were awarded the Nobel Prize in Chemistry 2019, for development of lithium-ion batteries).
He also states that “Yoshino could develop an efficient, working lithium-ion battery based on the ion transfer cell configuration. The identified carbonaceous material was thus used as an anode and Goodenough’s LixCoO2 material (typically containing small amounts of tin) was used as a cathode. Separator layers composed of polyethylene or polypropylene were used, and the electrolyte was composed of LiClO4 in propylene carbonate. In order to test the new configuration’s safety, Yoshino devised a testing unit by which a weight could be remotely dropped on the batteries”. Further studies on LIBs also suggested the varieties of materials for electrodes and electrolytes, which enhanced the efficiency for energy storage.
However, since lithium is a reactive metal, utmost care needs to be taken to protect this metal from air and water [6]. Therefore, the careful usage of lithium was of highest importance. For example, the incident of a Boeing airline catching fire due to the explosion of a lithium battery created a necessity to enhance the safety of LIBs [7].
Also, in the work reported by Kumar et al. [8], it was stated that due to the high costs of extraction and limited availability of the lithium reserves, the application of LIBs in large-scale energy storage systems had few constraints that need to be addressed. Especially due to the unequal spread of the lithium resources and scarcity of the deposits, the future application of LIBs seems to be challenged in terms of increased costs and concerns related to environment.
3. Recent studies on solid polymer-based batteries
In order to overcome completely the safety issues of LIBs, it was necessary to replace the conventional liquid electrolytes with other suitable materials, which could be used as electrolytes, while retaining the efficiency of the output parameters [9].

Figure 1. History of development in Polymer electrolytes (obtained from Zhang et al. [10])
In the early 70’s, the concept of polymer electrolytes (PEs) created a revolution in the battery technology by improving the aspects such as safety, durability, and flexibility. Numerous research studies have been done since then in order to increase the conductivity of these PEs, through selected and suitable dopant salts and plasticizer additives. The evolution and development of polymer electrolytes is shown in figure 1.
Few main advantages of polymer electrolytes (PEs) [11] were (i) low flammability (ii) ease of processing (iii) tolerant to mechanical deformation and (iv) better electrolyte-electrode contact. In the year 2020, Hager et al. [12] reported in their work that a special type of batteries (known as polymer-based batteries) were developed using organic materials as active components in the electrodes instead of using metals and metallic compounds as redox-active materials. The stated advantages of these batteries are shown in figure 2.

Figure 2. Advantages of polymer-based batteries
The genesis of the polymer batteries was the discovery of conducting polymers in 1970s, that led to an evergrowing demand for conducting polymers [12]. These polymer-based batteries displayed a variety of significant properties such as flexible fabrication of batteries and most importantly higher power densities. These were also found to be safe compared to LIBs [13]. However, these exhibited certain drawbacks, one being sloping of cell voltage. Eventually, the drawbacks of these were addressed through the addition of conducting additives such as carbon tubes or carbon nano fillers in order to enhance their conductivity.
Nearly 20 years after introducing the concept of PEs, researchers started to investigate and reported the preferred usage of “bio-based materials” as conventional host polymer materials, in place of synthetic polymers [14]. In their work, Rayung et al. [15] stated that “Even though most of the polymer electrolyte theories developed to date are based on synthetic materials, they hold true for bio-based polymers as well”.
Though synthetic polymers were preferred in replacing liquid electrolytes, the main issues to be addressed while using these are pollution and other environmental hazards, global warming, scarcity of resources, etc. These were addressed with the replacement of biopolymers as a nearly perfect renewable substitute to their counterparts.
4. Tamarind gum Biopolymer as a choice of biopolymer electrolyte
Based on the mechanism of extraction, synthesis, and origin of their source, bio-based polymers are polymers are classified into three main categories as shown in figure 3 [15].

Figure 3. Classification of biopolymers
Of the several stated biopolymers that were used as polymer electrolyte materials, Tamarind gum, also known as Tamarind seed polysaccharide (TSP) is one biopolymer that has potential usage in various sectors such as food, paper industry, textile industry, jute industry, and other industries. [16–18]. The schematic structure of the Tamarind seed polysaccharide is shown in figure 4.

Figure 4. Schematic representation of TSP (obtained from Chawananorasest et al. [19])
Though several stated uses of TSP were previously listed, the application of TSP based biopolymer electrolytes was initially reported in the work of Premalatha et al. [20]. The current article lists out the results from a series of research studies done using TSP as host biopolymer and a suitable sodium salt as the dopant material, for its application in storage devices.
- In 2022, Maithilee et al. [21] reported maximum ionic conductivity value of 1.7 × 10−3 S cm−1 for sodium-ion conducting biopolymers prepared using TSP as the host polymer and sodium perchlorate as the dopant salt. In the same year, Premalatha et al. [22] reported maximum ionic conductivity value of 1.23 × 10−3 S cm−1 for proton conducting biopolymers prepared using TSP as the host polymer and ammonium formate as the dopant salt.
- In 2023, Saha et al. [23] reported maximum ionic conductivity value of 1.94 × 10−4 S cm−1 for biopolymers prepared using TSP as the host polymer and sodium acetate as the dopant salt.
4.1. Role of Plasticizers in improving efficiency of PEs.
In 2001, Chandrasekaran et al. [24] have reported work on PEs based on PEO and NaClO3 with PEG as the plasticizer material, Na and MnO2 as the anode and cathode materials respectively. The results proved that addition of a suitable material (known as plasticizer) activated relaxations occurring in the polymer chain segments, that enabled ion hopping within the polymer. At room temperature, maximum conductivity of 9.47 × 10−4 S cm−1 was obtained.
In theory, through the addition of carbon nanotubes or fillers for a polymer will result in the formation of a network with high aspect ratio, which pave way to new pathways that are conducting, thereby enabling the overall conductivity through the reduction of interfacial resistance [25–27].
Based on similar results, research studies made through incorporation of ethylene carbonate plasticizer into the biopolymer salt complex proved an improved conductivity of the TSP biopolymer membranes. The details are given below.
- In 2023, Maithilee et al. [28] reported maximum ionic conductivity value of 1.49 × 10−3 S cm−1 for sodium-ion conducting biopolymers prepared using TSP as the host polymer and sodium nitrite as dopant salt
The summary of TSP based biopolymer electrolytes prepared using sodium as the dopant salt is tabulated below.

From the above examples, it was seen that when sodium salts were doped to TSP, conductivities of the order of 10-4 S cm-1 and 10-3 S cm-1 were obtained. Thrisha et al. [29] stated that the size and ion mobility play a very crucial role in the ionic conductivity of PEs. Salt doped PEs with ions of smaller sizes (like Na+ ions) exhibited greater conductivity. In the same work, it was stated that the interaction between the dopant salt and the host polymer can give rise to several amorphous regions, thereby reducing the crystallinity and increasing the conductivity. It was reported in their study that certain salts had the property of forming stable complexes while maintaining constant conductivity over time and others have not shown such property. Along with the choice of the dopant salts, the addition of suitable plasticizers and ionic liquids will further enhance the conductivity achieved [30].
In the preparation of polymer electrolytes, Rayung et al. [15] state that “for the construction of polymer electrolytes, several factors should be taken into consideration, including the choice of polymer host, the salts/acid dopants. The polymer host should possess certain characteristics such as good chemical, electrochemical, and photochemical stability, as well as good thermal and mechanical properties. Further, host polymers with a high concentration of polar groups (containing electron donors: O, NH, CN, F) are preferred. It is important to develop host polymers which have few crystalline phases and a relatively low glass transition temperature.”
5. Sodium as a substitute for Li batteries
The properties such as chemical, electrochemical and photochemical properties of synthetic polymers is well established in the research studies of several scientists [31–35]. However, for biopolymers such as TSP, recent research studies reported that they also exhibited good properties, as stated above. Sampathkumar et al. [36] have reported in their work that the property of electrochemical stability exhibited by TSP, to withstand fluctuations in the voltage and exhibit structural steadiness during the charge-discharge cycle, is one of the key factors in deciding the usage of TSP biopolymer electrolytes in batteries and supercapacitors. In terms of chemical stability, Monisha et al. [37] found that due to the nature of the origin and due to the power of resistance to decay in variety of environments exhibited by TSP, the electrochemical stability exhibited by TSP is in good comparison to synthetic polymers. Since TSP is not susceptible to chemical reactions, it is better suited for those applications where mild exposure of the material to reactive compounds such as acids or bases is expected. With respect to the property of photochemical stability, Malvia et al. [38] have reported that a substance is prone to photodegradation, when it contains a group of molecules called ‘chromophores’. This is also reported in the work of Dluga et al. [39]. From the work of Malvia et al., it is an indicative factor that TSP might show good photochemical stability. Thus, for optimum performance of a solid-state battery, the choice of biopolymers (like TSP) over synthetic polymers may be proposed and accepted.
The available choice of Sodium-ion batteries in comparison to Lithium-ion batteries are stated henceforth. An interesting fact from the 1870 novel “Twenty Thousand Leagues Under the Sea” by Jules Verne describes the submarine Nautilus powered by an advanced battery assembly unit. In the course of the novel, Captain Nemo mentions [40] that “…the cells with sodium must be regarded as most energetic, and that their electromotive force is double that of the zinc cells.” Though this was just mentioned on a casual note in the novel, the very first prototype of a sodium metal based solid-state battery was reported by Kumar et al. [8] which was fabricated and assembled by M. Armand, using the sodium metal as negative electrode, β-alumina as the solid electrolyte, and chromium oxide/ graphite intercalation compound (CrO3 @graphite) at the positive electrode in the year 1972. The use of preferring sodium as a substitute for lithium can be well understood through a careful observation of figure 5.

Figure 5. Differences between Li-ion and Na-ion batteries
(Source: https://www.iberdrola.com/documents/20125/3225538/baterias-iones-sodio-infografia-EN.pdf)
Compared to the extensively-used lithium-ion (Li-ion) batteries, Na-ion batteries have a lower energy density and cycle life, but they perform better in a wide operational temperature range and are safer. Na-ion cells have a similar working principle to Li-ion cells and are expected to be at least 20% cheaper than LFP due to their lithium-free nature [41,42]. However, separator and electrolyte costs could be significant and result in Na-ion being more costly. Figure 6 shows the comparison between Li-ion and Na-ion batteries.
A sodium battery is basically a battery that deploys Na+ ions as the charge carriers. The working principle of this battery is similar to that of LIB except the fact that lithium ions are replaced by sodium ions [43].

Figure 6. Comparison between Li-ion and Na-ion batteries
(Source: Wood Mackenzie https://www.woodmac.com/news/opinion/will-sodium-ion-battery-cells-be-a-game-changer-
for-electric-vehicle-and-energy-storage-markets)
Current studies are addressing the minor drawbacks of sodium ion batteries, one such being lower energy density [44,45]. However, the choice of sodium comes from the fact that elements belonging to the same group exhibit similar chemical properties. The added advantages of these over lithium batteries paved way to the commercialization of sodium batteries.
6. Conclusion
In the recent work of Kumar et al. [8], the best suited polymers for Na-ion batteries are depicted in Figure 7.
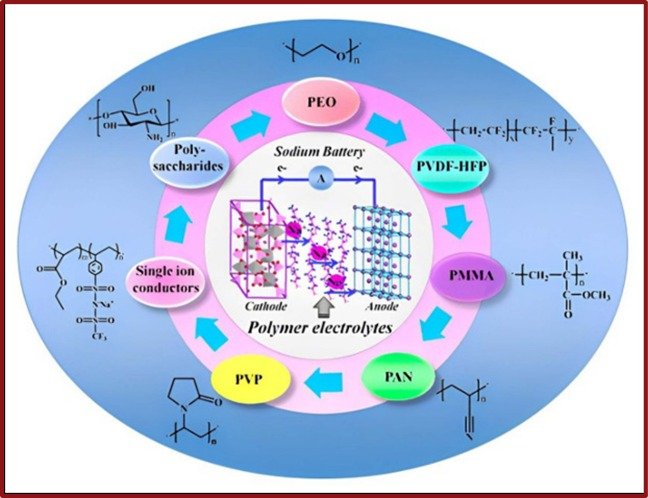
Figure 7. Polymer Electrolytes for Sodium batteries (obtained from Kumar et al. [8])
Copyright 2019, American Chemical Society
Although there are certain limitations of biopolymers, such as relatively weaker mechanical strengths [46] and challenges in processing [47], there are potential advantages these offer, such as biocompatibility, sustainability, biodegradable nature and ecological nature. Therefore, these are preferred in comparison to synthetic polymers [48–50]. Thus, for the fabrication of a solid-state battery, the preference of using TSP biopolymer as a substitute to the conventional synthetic polymers is preferred [51,52]. Apart from this, the preference of sodium metal in place of lithium metal, from a perspective that in the coming times, biopolymer-based sodium batteries will rule the future of modern-day solid-state battery technology. Though extensive ongoing research is focused on in the applications of Na-ion batteries in place of lithium batteries, the choice of preferring sodium ion batteries is mainly due to their cost-effectiveness, recyclability, and better performance even at lower temperatures as compared to lithium batteries [53]. Resolving the issues like the energy density of sodium batteries and enhancement of the conductivity of biopolymer electrolytes will definitely be considered as suitable solutions for large scale production of these batteries with minimum cost [54]..
Author Contributions: Conceptualization - P.V.N.M and N.K.J; Methodology - M.S.J; writing—original draft preparation – P.V.N.M, A.S.R and R.M; writing—review and editing – P.V.N.M, N.K.J and M.S.J;
Funding: This research received no external funding.
Acknowledgments: The authors are grateful to the University for enabling them to pursue research in this field.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- A. Riaz, M.R. Sarker, M.H.M. Saad, R. Mohamed, Review on Comparison of Different Energy Storage Technologies Used in Micro-Energy Harvesting, WSNs, Low-Cost Microelectronic Devices: Challenges and Recommendations, Sensors 21 (2021) 5041. https://doi.org/10.3390/s21155041.
- E. Hossain, H. Faruque, Md. Sunny, N. Mohammad, N. Nawar, A Comprehensive Review on Energy Storage Systems: Types, Comparison, Current Scenario, Applications, Barriers, and Potential Solutions, Policies, and Future Prospects, Energies 13 (2020) 3651. https://doi.org/10.3390/en13143651.
- H.B. Glushakow, In Search of an Energy Miracle, in: 2021 4th International Conference on Energy Conservation and Efficiency (ICECE), IEEE, Lahore, Pakistan, 2021: pp. 1–6. https://doi.org/10.1109/ ICECE51984.2021.9406305.
- E.G. Garrison, A history of engineering and technology: artful methods, 2nd ed, CRC Press, Boca Raton, Fla, 1999.
- K. Grandin, The Nobel Prizes 2019, WORLD SCIENTIFIC, 2022. https://doi.org/10.1142/12826.
- N. Bolan, S.A. Hoang, M. Tanveer, L. Wang, S. Bolan, P. Sooriyakumar, B. Robinson, H. Wijesekara, M. Wijesooriya, S. Keerthanan, M. Vithanage, B. Markert, S. Fränzle, S. Wünschmann, B. Sarkar, A. Vinu, M.B. Kirkham, K.H.M. Siddique, J. Rinklebe, From mine to mind and mobiles – Lithium contamination and its risk management, Environmental Pollution 290 (2021) 118067. https://doi.org/10.1016/j.envpol.2021.118067.
- N. Williard, W. He, C. Hendricks, M. Pecht, Lessons Learned from the 787 Dreamliner Issue on Lithium- Ion Battery Reliability, Energies 6 (2013) 4682–4695. https://doi.org/10.3390/en6094682.
- S. Kumar, R. Raghupathy, M. Vittadello, Sodium Polymer Electrolytes: A Review, Batteries 10 (2024) 73. https://doi.org/10.3390/batteries10030073.
- M. Pigłowska, B. Kurc, M. Galiński, P. Fuć, M. Kamińska, N. Szymlet, P. Daszkiewicz, Challenges for Safe Electrolytes Applied in Lithium-Ion Cells—A Review, Materials 14 (2021) 6783. https://doi.org/10.3390/ ma14226783.
- D. Zhang, S. Li, Q. Xiong, Z. Huang, H. Hong, S. Yang, J. Zhu, C. Zhi, Interface challenges and research progress toward solid polymer electrolytes-based lithium metal batteries, MetalMat 1 (2024) e13. https:// doi.org/10.1002/metm.13.
- Y. An, X. Han, Y. Liu, A. Azhar, J. Na, A.K. Nanjundan, S. Wang, J. Yu, Y. Yamauchi, Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond, Small 18 (2022) 2103617. https://doi. org/10.1002/smll.202103617.
- M.D. Hager, B. Esser, X. Feng, W. Schuhmann, P. Theato, U.S. Schubert, Polymer-Based Batteries—Flexible and Thin Energy Storage Systems, Advanced Materials 32 (2020) 2000587. https://doi.org/10.1002/ adma.202000587.
- J. Kalhoff, G.G. Eshetu, D. Bresser, S. Passerini, Safer Electrolytes for Lithium-Ion Batteries: State of the Art and Perspectives, ChemSusChem 8 (2015) 2154–2175. https://doi.org/10.1002/cssc.201500284.
- A. Kumar, G. Sharma, Mu. Naushad, A.H. Al-Muhtaseb, A. García-Peñas, G.T. Mola, C. Si, F.J. Stadler, Bio- inspired and biomaterials-based hybrid photocatalysts for environmental detoxification: A review, Chemical Engineering Journal 382 (2020) 122937. https://doi.org/10.1016/j.cej.2019.122937.
- M. Rayung, M.M. Aung, S.C. Azhar, L.C. Abdullah, M.S. Su’ait, A. Ahmad, S.N.A.M. Jamil, Bio-Based Polymer Electrolytes for Electrochemical Devices: Insight into the Ionic Conductivity Performance, Materials 13 (2020) 838. https://doi.org/10.3390/ma13040838.
- B.B. Mansingh, J.S. Binoj, N.P. Sai, S.A. Hassan, S. Siengchin, M.R. Sanjay, Y.C. Liu, Sustainable development in utilization of Tamarindus indica L. and its by-products in industries: A review, Current Research in Green and Sustainable Chemistry 4 (2021) 100207. https://doi.org/10.1016/j.crgsc.2021.100207.
- C.K. Nagar, S.K. Dash, K. Rayaguru, Tamarind seed: Composition, applications, and value addition: A comprehensive review, Food Processing Preservation 46 (2022). https://doi.org/10.1111/jfpp.16872.
- H. Zhang, H. Cui, F. Xie, Z. Song, L. Ai, Tamarind seeds polysaccharide: Structure, properties, health benefits, modification and food applications, Food Hydrocolloids (2024) 110222. https://doi.org/10.1016/j. foodhyd.2024.110222.
- K. Chawananorasest, P. Saengtongdee, P. Kaemchantuek, Extraction and Characterization of Tamarind (Tamarind indica L.) Seed Polysaccharides (TSP) from Three Difference Sources, Molecules 21 (2016) 775. https://doi.org/10.3390/molecules21060775.
- M. Premalatha, T. Mathavan, S. Selvasekarapandian, S. Monisha, D.V. Pandi, S. Selvalakshmi, Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate, Journal of Non-Crystalline Solids 453 (2016) 131–140. https://doi.org/10.1016/j. jnoncrysol.2016.10.008.
- K. Maithilee, P. Sathya, S. Selvasekarapandian, R. Chitra, M.V. Krishna, S. Meyvel, Na-ion conducting biopolymer electrolyte based on tamarind seed polysaccharide incorporated with sodium perchlorate for primary sodium-ion batteries, Ionics 28 (2022) 1783–1790. https://doi.org/10.1007/s11581-022-04440-7.
- M. Premalatha, S. Monisha, S. Selvalakshmi, T. Mathavan, V. Moniha, Investigation of hydrogen ion transport in NH4HCO2 doped TSP biopolymer electrolyte for battery applications, Materials Letters 320 (2022) 132369. https://doi.org/10.1016/j.matlet.2022.132369.
- A. Saha, K.V. Kumar, N.K. Jyothi, M.G. Kiran, M.C. Rao, Structural and electrical properties of TSP: CH3COONa amorphous Biopolymer electrolytes for electrochemical cell applications, Journal of Non- Crystalline Solids 616 (2023) 122465. https://doi.org/10.1016/j.jnoncrysol.2023.122465.
- R. Chandrasekaran, S. Selladurai, Preparation and characterization of a new polymer electrolyte (PEO:NaClO3) for battery application, J Solid State Electrochem 5 (2001) 355–361. https://doi.org/10.1007/ s100080000156.
- Y. Gao, F. Qu, W. Wang, F. Li, X. Zhao, L. Zhang, Increasing the electrical conductivity of polymer nanocomposites under the external field by tuning nanofiller shape, Composites Science and Technology 176 (2019) 37–45. https://doi.org/10.1016/j.compscitech.2019.03.025.
- M. Ikram, A. Raza, A. Shahbaz, H. Ijaz, S. Ali, A. Haider, M. Tayyab Hussain, J. Haider, A. Ahmed Rafi, S. Ali, Carbon Nanotubes, in: M. Ikram, A. Maqsood (Eds.), 21st Century Advanced Carbon Materials for Engineering Applications - A Comprehensive Handbook, IntechOpen, 2021. https://doi.org/10.5772/ intechopen.95442.
- A. Chanda, S.K. Sinha, N.V. Datla, Electrical conductivity of random and aligned nanocomposites: Theoretical models and experimental validation, Composites Part A: Applied Science and Manufacturing 149 (2021) 106543. https://doi.org/10.1016/j.compositesa.2021.106543.
- K. Maithilee, P. Sathya, S. Selvasekarapandian, R. Chitra, S. Meyvel, Investigation on tamarind seed polysaccharide biopolymer electrolyte doped with sodium nitrite and EC plasticizer for primary sodium battery, Bull Mater Sci 46 (2023) 114. https://doi.org/10.1007/s12034-023-02948-w.
- Thrisha K, Saratha R, Natural polymer-based electrolytes for energy storage devices—an overview, Ionics 30 (2024) 1245–1266. https://doi.org/10.1007/s11581-023-05315-1.
- F.C. Tavares, C.M. Cholant, E.C. Kohlrausch, G. R. Bolzan, P.F.B. Gonçalves, E.S. Gil, S. Khan, J. Dupont,C.O. Avellaneda, M.J. Leite Santos, Ionic Liquid Boosted Conductivity of Biopolymer Gel Electrolyte, J. Electrochem. Soc. 170 (2023) 084501. https://doi.org/10.1149/1945-7111/ace937.
- T. Celiker, R. İsci, K. Kaya, T. Ozturk, Y. Yagci, Photoinduced STEP-GROWTH polymerization of thieno[3,4-b] thiophene derivatives. The substitution effect on the reactivity and electrochemical properties, Journal of Polymer Science 58 (2020) 2327–2334. https://doi.org/10.1002/pol.20200398.
- J. Liu, L. Lu, D. Wood, S. Lin, New Redox Strategies in Organic Synthesis by Means of Electrochemistry and Photochemistry, ACS Cent. Sci. 6 (2020) 1317–1340. https://doi.org/10.1021/acscentsci.0c00549.
- D. Hao, Y. Liu, S. Gao, H. Arandiyan, X. Bai, Q. Kong, W. Wei, P.K. Shen, B.-J. Ni, Emerging artificial nitrogen cycle processes through novel electrochemical and photochemical synthesis, Materials Today 46 (2021) 212–233. https://doi.org/10.1016/j.mattod.2021.01.029.
- G. Singh, S. Chandra, Unravelling the structural-property relations of porphyrinoids with respect to photo- and electro-chemical activities, Electrochemical Science Adv 3 (2023) e2100149. https://doi.org/10.1002/ elsa.202100149.
- N. Singh, U. Riaz, Recent trends on synthetic approaches and application studies of conducting polymers and copolymers: a review, Polym. Bull. 79 (2022) 10377–10408. https://doi.org/10.1007/s00289-021- 03987-1.
- L. Sampathkumar, P. Christopher Selvin, S. Selvasekarapandian, P. Perumal, R. Chitra, M. Muthukrishnan, Synthesis and characterization of biopolymer electrolyte based on tamarind seed polysaccharide, lithium perchlorate and ethylene carbonate for electrochemical applications, Ionics 25 (2019) 1067–1082. https:// doi.org/10.1007/s11581-019-02857-1.
- S. Monisha, P. Prameela, G. Boopathi, S. Selvalakshmi, S. Gnanam, J. Gajendiran, Synthesis, structural properties, thermal behavior, and electrical and electrochemical sensing performance of tamarind seed polysaccharide-lithium nitrate polymer electrolyte, Ionics 30 (2024) 2807–2818. https://doi.org/10.1007/ s11581-024-05439-y.
- R. Malviya, S. Sundram, S. Fuloria, V. Subramaniyan, K.V. Sathasivam, A.K. Azad, M. Sekar, D.H. Kumar, S. Chakravarthi, O. Porwal, D.U. Meenakshi, N.K. Fuloria, Evaluation and Characterization of Tamarind Gum Polysaccharide: The Biopolymer, Polymers 13 (2021) 3023. https://doi.org/10.3390/polym13183023.
- A. Długa, D. Bajer, H. Kaczmarek, Photochemical and Thermal Stability of Bionanocellulose/Poly(Vinyl Alcohol) Blends, Polymers 14 (2022) 4364. https://doi.org/10.3390/polym14204364.
- Z. Song, F. Chen, M. Martinez-Ibañez, W. Feng, M. Forsyth, Z. Zhou, M. Armand, H. Zhang, A reflection on polymer electrolytes for solid-state lithium metal batteries, Nat Commun 14 (2023) 4884. https://doi. org/10.1038/s41467-023-40609-y.
- M. Li, H. Zhuo, Q. Jing, Y. Gu, Z. Liao, K. Wang, J. Hu, D. Geng, X. Sun, B. Xiao, Low-temperature performance of Na-ion batteries, Carbon Energy 6 (2024) e546. https://doi.org/10.1002/cey2.546.
- S.S.A. Kumar, M. Nujud Badawi, J. Liew, T. Prasankumar, K. Ramesh, S. Ramesh, S. Ramesh, S.K. Tiong, High-Performance Sodium-Ion Batteries with Graphene: An Overview of Recent Developments and Design, ChemSusChem (2024) e202400958. https://doi.org/10.1002/cssc.202400958.
- N. Tapia-Ruiz, A.R. Armstrong, H. Alptekin, M.A. Amores, H. Au, J. Barker, R. Boston, W.R. Brant, J.M. Brittain, Y. Chen, M. Chhowalla, Y.-S. Choi, S.I.R. Costa, M. Crespo Ribadeneyra, S.A. Cussen, E.J. Cussen, W.I.F. David, A.V. Desai, S.A.M. Dickson, E.I. Eweka, J.D. Forero-Saboya, C.P. Grey, J.M. Griffin, P. Gross, X. Hua, J.T.S. Irvine, P. Johansson, M.O. Jones, M. Karlsmo, E. Kendrick, E. Kim, O.V. Kolosov, Z. Li, S.F.L. Mertens, R. Mogensen, L. Monconduit, R.E. Morris, A.J. Naylor, S. Nikman, C.A. O’Keefe, D.M.C. Ould, R.G. Palgrave, P. Poizot, A. Ponrouch, S. Renault, E.M. Reynolds, A. Rudola, R. Sayers, D.O. Scanlon, S. Sen, V.R. Seymour, B. Silván, M.T. Sougrati, L. Stievano, G.S. Stone, C.I. Thomas, M.-M. Titirici, J. Tong, T.J. Wood, D.S. Wright, R. Younesi, 2021 roadmap for sodium-ion batteries, J. Phys. Energy 3 (2021) 031503. https://doi.org/10.1088/2515-7655/ac01ef.
- K. Mathiyalagan, D. Shin, Y.-C. Lee, Difficulties, strategies, and recent research and development of layered sodium transition metal oxide cathode materials for high-energy sodium-ion batteries, Journal of Energy Chemistry 90 (2024) 40–57. https://doi.org/10.1016/j.jechem.2023.10.023.
- J. Chen, G. Adit, L. Li, Y. Zhang, D.H.C. Chua, P.S. Lee, Optimization Strategies Toward Functional Sodium- Ion Batteries, Energy & Environ Materials 6 (2023) e12633. https://doi.org/10.1002/eem2.12633.
- K.M. Zia, N. Akram, S. Tabasum, A. Noreen, M.U. Akbar, Processing of bio-based polymers, in: Processing Technology for Bio-Based Polymers, Elsevier, 2021: pp. 151–189. https://doi.org/10.1016/B978-0-323- 85772-7.00003-3.
- T. Gurunathan, S. Mohanty, S.K. Nayak, A review of the recent developments in biocomposites based on natural fibres and their application perspectives, Composites Part A: Applied Science and Manufacturing 77 (2015) 1–25. https://doi.org/10.1016/j.compositesa.2015.06.007.
- L. Manfra, V. Marengo, G. Libralato, M. Costantini, F. De Falco, M. Cocca, Biodegradable polymers: A real opportunity to solve marine plastic pollution?, Journal of Hazardous Materials 416 (2021) 125763. https:// doi.org/10.1016/j.jhazmat.2021.125763.
- S. Walker, R. Rothman, Life cycle assessment of bio-based and fossil-based plastic: A review, Journal of Cleaner Production 261 (2020) 121158. https://doi.org/10.1016/j.jclepro.2020.121158.
- G. Satchanska, S. Davidova, P.D. Petrov, Natural and Synthetic Polymers for Biomedical and Environmental Applications, Polymers 16 (2024) 1159. https://doi.org/10.3390/polym16081159.
- J. Ding, Y. Yang, J. Poisson, Y. He, H. Zhang, Y. Zhang, Y. Bao, S. Chen, Y.M. Chen, K. Zhang, Recent Advances in Biopolymer-Based Hydrogel Electrolytes for Flexible Supercapacitors, ACS Energy Lett. 9 (2024) 1803–1825. https://doi.org/10.1021/acsenergylett.3c02567.
- N. Borah, A. Kar, N. Karak, Biocomposites of biopolymers with metals and their derivatives, in: Advances in Biocomposites and Their Applications, Elsevier, 2024: pp. 167–200. https://doi.org/10.1016/B978-0-443- 19074-2.00006-X.
- C. Vaalma, D. Buchholz, M. Weil, S. Passerini, A cost and resource analysis of sodium-ion batteries, Nat Rev Mater 3 (2018) 18013. https://doi.org/10.1038/natrevmats.2018.13.
- W. Zhao, M. Wang, H. Lin, K. Kim, R. He, S. Feng, H. Liu, Research progress on electrolyte key salts for sodium-ion batteries, Progress in Natural Science: Materials International 34 (2024) 263–273. https://doi. org/10.1016/j.pnsc.2024.03.003.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of SSSUHE and/or the editor(s). SSSUHE and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content.

